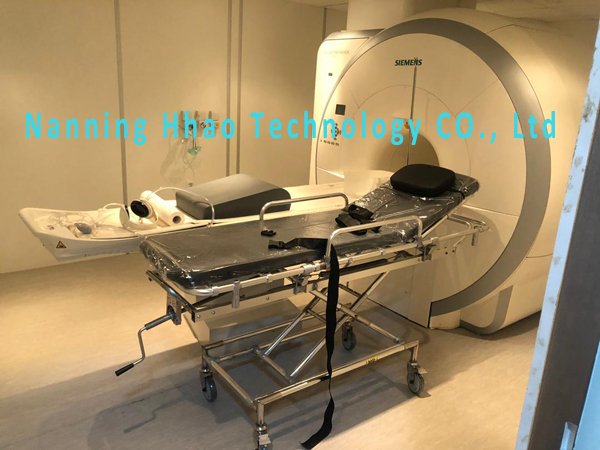
MRI compatible stretcher trolley/ height adjustable/ for 1.5T and 3.0T
Magentic test by a hand held magnet:
The MRI Conditional Adjustable Height Stretcher is fully tested to 3T.
Certificates: CE ( MDR 2017/745), EU free sales certificate, Italy SFDA registration certificate.
Application: NMR, CT room, Veterinary hospital
MR conditional to 1.5T and 3.0T SIEMENS, GE, PHILIPS, TOSHIBA, CANON, HITACH
Main Features:
Aluminum construction
Drop down side rails
5" Lock Casters, 2 with brake, 2 without brake
Adjustable height
Adjustable headrest
2.8" pad included
Main Specifications
Max Height:: 33” (189cm)
Min Height: 11.8” (46cm)
Overall Length: 74” (189cm)
Overall Width: 22” (56cm)
Back Adj. Range: 0--75 degree
Max loading Capacity: 373 lbs ( 169kgs)
Warranty:
2 years on frame
1 year on soft goods and moving parts
CERTIFICATES: CE with MDR 2017/745 + Italy SFDA registration certificate:

Contact: Sarah
Phone: +86-13737941709
Tel: +86-771-6759549
Email: Sarah@hhao-tech.com
Add: FLAT/RM A17,9/F SILVERCORP INT'L TOWER 707+713 NATHAN RD MONGKOK KLN HONG KONG